NGOtiate is a Danish consultancy house
We are a rare hybrid and a pioneer in our field
About us
NGOtiate is a Danish consultancy house founded in 2007. We are a rare hybrid and a pioneer in our field:
Our services
be the change
Helping patient organizations, advocacy groups and digital communities
- Stakeholder and communication strategies and development of implementation plans
- Design of related digital communication, marketing or fundraising initiatives
- Day-to-day strategic and operational support towards defined goals
- Knowledge of how to partner with research-based organizations, in order to pave way for new medicines development in your area of interest and related match-finding
Helping research-based organizations
- Introduction to strategic patient engagement and related benefits principles, practice, guidelines and critical success factors
- Patient engagement strategy development – corporate level, portfolio level or compound specific
- Applied strategic patient engagement/increase specific or overall performance across and beyond the value chain
- Development of internal essential infrastructure (including or excluding related training by subject matter experts)
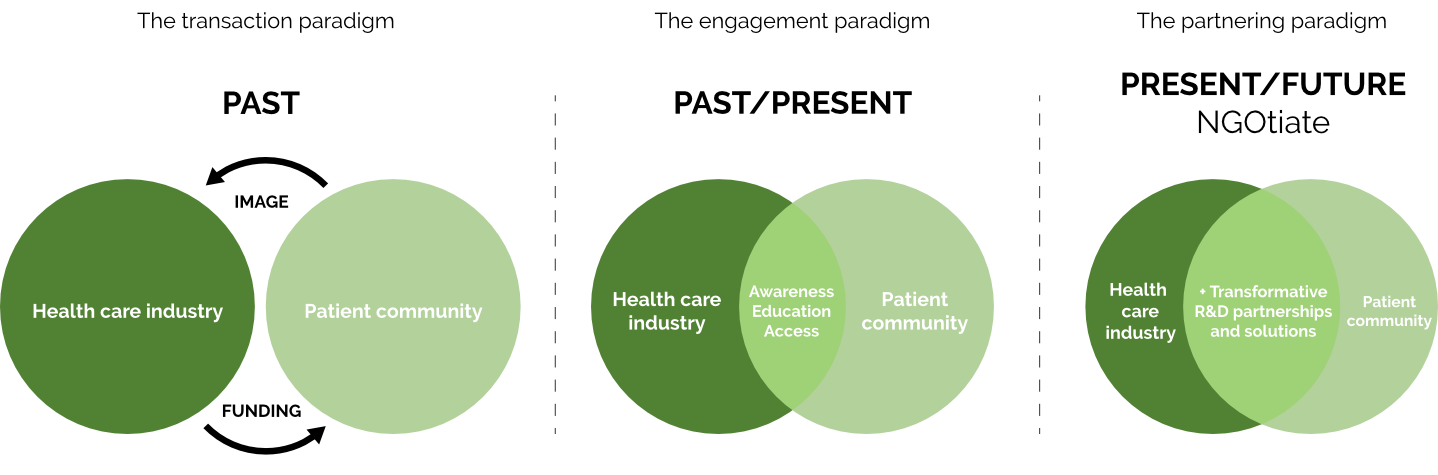
HOW WE ARE DIFFERENT
the partnering paradigm
Sources: Eupati.eu, fda.gov and EMA Europe
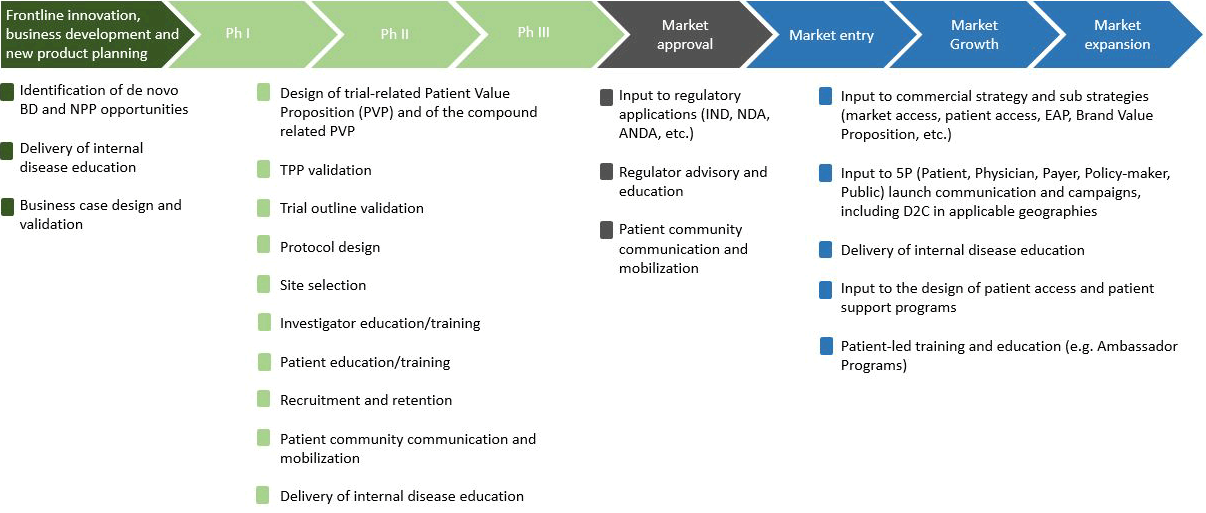
The value of strategic patient engagement
- Optimizing performance across value chain and ultimately end-user products (ref 1,6)
- Positive effect (e.g. financial effect) on clinical trial recruitment and retention (ref 2,3,4)
- Opportunity for researchers to learn from patients as disease experience experts (ref 6)
- Challenge the set way of thinking – often leading to improved research design, delivery, and dissemination (ref 6)
- Wider impacts of a changed innovation, research culture and agenda (ref 5)
Sources:
1: Wicks P, Richards T, Denegri S, Godlee F. Patients’ roles and rights in research.
2: Crocker Joanna C, Ricci-Cabello Ignacio, Parker Adwoa, Hirst Jennifer A, Chant Alan, Petit-Zeman Sophie et al. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis
3: Boivin Antoine, Richards Tessa, Forsythe Laura, Grégoire Alexandre, Esperance Audrey, Abelson Julia et al. Evaluating patient and public involvement in research.
4: Levitan, Getz et al. 2018: Assessing the Financial Value of Patient Engagement 5 Staley, K. “Researchers don’t know what they’re missing”—the impact of patient involvement in research
6: Vat et. al (2019) Evaluating the “return on patient engagement initiatives” in medicines research and development